SAIZEN®
[SOMATROPIN (rDNA ORIGIN) FOR INJECTION]
About Saizen
Saizen® is a brand of growth hormone made by EMD Serono Inc.. It is used to replace the growth hormone that is missing in a person’s body. Saizen ® is currently FDA-approved for treating children or adults with growth hormone deficiency (GHD).
Saizen ® is given as an injection. When you order Saizen ®, Ocean Breeze Healthcare will supply you with all the necessary supplies you would need to administer the Saizen®.
Saizen ® is given as an injection. When you order Saizen ®, Ocean Breeze Healthcare will supply you with all the necessary supplies you would need to administer the Saizen®.
What form is Saizen® currently available?
Saizen® is currently available in 2 forms: a multi-dose dry-powder vial and cartridge.
The vial is available in 5 mg and 8.8 mg strength. The dry powder drug vial needs to be reconstituted, or mixed with the diluent (the clear liquid solution) provided in the same box as the drug vial.
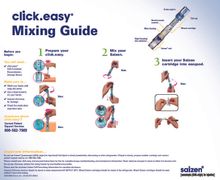
Cartridge is 8.8 mg strength. Cartridges come with either a click. easy® device or saizenprep® device used for mixing the medication.
The vial is available in 5 mg and 8.8 mg strength. The dry powder drug vial needs to be reconstituted, or mixed with the diluent (the clear liquid solution) provided in the same box as the drug vial.
Cartridge is 8.8 mg strength. Cartridges come with either a click. easy® device or saizenprep® device used for mixing the medication.
What are some potential side effect of Saizen®?
Saizen® is generally well tolerated. While unlikely, potential side effects include injection site reaction, such as rash, redness or bruising, and headaches. Some may experience symptoms of swelling, joint pains, or high blood sugar. In rare cases, pancreatitis have occurred, be sure to report any incidence of severe abdominal pain.
If you are experiencing any unwanted side effect, contact your doctor or pharmacist. You may also report the side effect to the FDA at www.fda.gov/medwatch, or call 1-800-FDA-1088.
If you are experiencing any unwanted side effect, contact your doctor or pharmacist. You may also report the side effect to the FDA at www.fda.gov/medwatch, or call 1-800-FDA-1088.
How do you administer Saizen®?
Saizen® is injected subcutaneously. Both the vials and the cartridges can be administered using either easypod® or one. click® pen device.
For Vials:
Saizen® can be injected with regular syringe with needle or using either the easypod® device or one.click® provided by EMD Serono, Inc. Saizen® must be mixed with the provided mixing liquid, or the diluent, before injection. Your prescription will tell you how much liquid to add into the Saizen® powder vial. Swirl the vial gently until the content is completely dissolved. Do not shake the vial. administration instruction, please refer to “preparation and administration” section of the Saizen® Prescriber Information (PDF saizen PI 04_2012). For more information on using Saizen® vial with easypod®, please read (PDF: SAIZEN SAIZEN EASYPOD INSTRUCTIONS FOR USE). Forinformation about using Saizen® vial with cool.click™2 device, please read instruction manual (PDF: cool.click instuction manual).
For Cartridge:
Saizen® cartridge is administered using easypod® or one.click®. For easypod® and one. click®, 8.8 mg vials are reconstituted using eitherclick. easy® (for existing patients) or saizenprep® (for new patients as of May 1, 2017).
For Vials:
Saizen® can be injected with regular syringe with needle or using either the easypod® device or one.click® provided by EMD Serono, Inc. Saizen® must be mixed with the provided mixing liquid, or the diluent, before injection. Your prescription will tell you how much liquid to add into the Saizen® powder vial. Swirl the vial gently until the content is completely dissolved. Do not shake the vial. administration instruction, please refer to “preparation and administration” section of the Saizen® Prescriber Information (PDF saizen PI 04_2012). For more information on using Saizen® vial with easypod®, please read (PDF: SAIZEN SAIZEN EASYPOD INSTRUCTIONS FOR USE). Forinformation about using Saizen® vial with cool.click™2 device, please read instruction manual (PDF: cool.click instuction manual).
For Cartridge:
Saizen® cartridge is administered using easypod® or one.click®. For easypod® and one. click®, 8.8 mg vials are reconstituted using eitherclick. easy® (for existing patients) or saizenprep® (for new patients as of May 1, 2017).
How do you store Saizen®?
Before, reconstitution, Saizen® can be stored in room temperature. After reconstitution, Saizen® must be stored in the refrigerator (at 2 – 8° Celsius)at all times.
How long does Saizen® last?
Saizen® is stable and last until the indicated expiration date before opening.
Saizen® 5 mg and 8.8 mg vials reconstituted with the Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) provided should be stored under refrigeration (36°–46°F/2°–8°C) for up to 14 days.
Saizen® 8.8 mg cartridge reconstituted with the Sterile Water for Injection, 0.3% (w/v) metacresol provided should be stored under refrigeration(36°–46°F/2°–8°C) for up to 21 days. Avoid freezing reconstituted vials or cartridges of Saizen®.
Saizen® 5 mg and 8.8 mg vials reconstituted with the Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) provided should be stored under refrigeration (36°–46°F/2°–8°C) for up to 14 days.
Saizen® 8.8 mg cartridge reconstituted with the Sterile Water for Injection, 0.3% (w/v) metacresol provided should be stored under refrigeration(36°–46°F/2°–8°C) for up to 21 days. Avoid freezing reconstituted vials or cartridges of Saizen®.
Is there preservatives in the Saizen®?
Yes, Saizen® cartridge contains metacresol and Saizen® vial contains benzyl alcohol.
How often do I take the Saizen®?
You should take the Saizen® exactly as prescribed by your doctor. In general, Saizen® is given as a once-a-day injection.
Manufacturer Website
www.saizenus.com
Information on this site is provided for informational purposes and is not meant to substitute the advice provided by your own physician or other medical professional. You should not use the information contained herein for diagnosing or treating a health problem or disease, or prescribing any medication. You should read carefully all product packaging. If you have or suspect that you have a medical problem, promptly contact your health care provider. Pursuant to 21 U.S.C. § 333 (e) (1), which limits usage only to the treatment of disease or other recognized medical conditions authorized by the Secretary of Health and Human Services, Ocean Breeze Healthcare does not dispense human growth hormone for anti-aging, cosmetic or performance enhancement purposes.
www.saizenus.com
Information on this site is provided for informational purposes and is not meant to substitute the advice provided by your own physician or other medical professional. You should not use the information contained herein for diagnosing or treating a health problem or disease, or prescribing any medication. You should read carefully all product packaging. If you have or suspect that you have a medical problem, promptly contact your health care provider. Pursuant to 21 U.S.C. § 333 (e) (1), which limits usage only to the treatment of disease or other recognized medical conditions authorized by the Secretary of Health and Human Services, Ocean Breeze Healthcare does not dispense human growth hormone for anti-aging, cosmetic or performance enhancement purposes.